2. 土壤与农业可持续发展国家重点实验室(中国科学院南京土壤研究所), 南京 210008
铁是所有生物生命活动所必需的微量元素,和人类健康息息相关。缺铁性贫血症是全球最严重的健康问题之一,影响约20亿人口,我国由于铁等微量元素摄取不足导致的“隐形饥饿”人口多达3亿。摄取富含铁等的农产品是远离“隐形饥饿”,预防缺铁性贫血症最安全、经济、有效的方法,这对于山区和贫困地区的人们尤为重要。培育富铁植物的前提是揭示植物铁稳态的分子调控机制。作为辅酶因子和电子传递链关键组分,铁参与了植物光合作用、呼吸作用、氮固定、氨基酸合成等多种重要的生理代谢过程[1-5]。缺铁导致叶片黄化,植物生长发育严重受阻,产量和品质显著降低。铁在地壳中的含量虽然很丰富,但在高pH和通气良好的土壤中大多以溶解度极低的Fe3+氧化态形式存在,植物不能直接吸收利用,限制了铁在土壤中的生物有效性,导致植物生长发育受阻,致使植物缺铁失绿已成为全世界普遍关注的问题。据统计,全世界约有40% 的土壤缺乏生物有效性铁,特别是在石灰性土壤上,许多农作物常因发生缺铁失绿导致生长不良,产量和品质下降,进而影响人们的营养健康。相反在酸性和长期淹水逆境下,土壤中Fe2+含量大大增加,导致植物体内吸收累积过量的铁,通过芬顿化学过程铁和氧气反应产生大量的活性氧类物质,导致植物伤害甚至死亡[1]。因此,植物必须严格调控细胞内的铁稳态,既避免不足也要防止过量,达到对植物生长发育最优化。
1 策略Ⅰ植物铁的吸收机制虽然铁在地壳中是含量第四丰富的矿质元素,但在中性或碱性通气良好的土壤中,铁以植物难以直接利用的难溶态复合物形式存在,自由态的Fe3+和Fe2+的浓度通常小于10–15 mol/L,远远低于适于植物生长发育所需的浓度10–9 ~ 10–4 mol/L[6]。铁又是叶绿素合成所必需的微量元素,但其在植物体内不易移动,因此缺铁典型症状就是新叶黄化泛白。在长期的进化过程中,固着生长的植物形成了两种铁吸收机制来适应缺铁环境,也即通常所指的机制Ⅰ和机制Ⅱ[7]。禾本科植物,如水稻、玉米、小麦等采用机制Ⅱ(strategy Ⅱ)来响应缺铁胁迫。缺铁后根系合成大量的植物铁载体(phytosiderophores, PS),并由转运蛋白TOM(transporter of mugineic acid)分泌到根际[8],与土壤中的Fe3+螯合,再由定位在根细胞质膜上的转运蛋白YS1(yellow strip1)及其同源蛋白YSL(yellow strip1 like)直接将铁螯合物转运至细胞内[3, 6, 9]。双子叶和非禾本科单子叶植物,如番茄、果树和模式植物拟南芥,采用机制Ⅰ(strategyⅠ)来应对缺铁胁迫,因此这些植物往往也被称为策略Ⅰ植物。策略Ⅰ植物通常有3个协同关联的机制来应对缺铁时对铁的高效吸收,这也是涉及细胞铁稳态的第一步和关键环节。植物感受缺铁后,首先是根系H+-ATPase活性增强,分泌更多质子,降低土壤pH,增加根际土壤中铁的溶解[10];伴随H+-ATPase活性增强的同时,根系分泌香豆素类(秦皮素、菱黄碱)和核黄素类次级代谢物来增加Fe3+的移动性[11-14],随之,三价铁还原酶FRO2(ferric reduction oxidase 2)转录表达升高、活性增强,将Fe3+还原成Fe2+[15];最后大量表达的高亲和性二价铁转运蛋白IRT1(iron-regulated transporter 1)将亚铁离子转运到细胞内[3, 6, 16]。特别值得强调的是,最近研究发现,缺铁时除了质子大量分泌外,拟南芥缺铁时同时分泌高亲和性的酚类铁螯合物来应对缺铁胁迫,特别是在高pH条件下,这些次级代谢产物的分泌是拟南芥响应缺铁胁迫所必需的[14]。介导香豆素类化合物从胞内分泌到胞外主要是由转运蛋白PDR9/ABCG37(pleiotropic drug resistance 9/ATP- binding cassette G37)完成的[11-17]。
2 策略Ⅰ植物铁的转运机制植物根系吸收铁以后,必须进行正确的转运和分配,将铁运输到植物各个需要的部位,完成生理功能或将铁储存起来。目前普遍接受的观点是根系吸收的铁需要与柠檬酸盐螯合后,通过木质部导管将铁运输到地上部,柠檬酸分泌载体属于MATE (multidrug and toxic compound extrusion) 家族蛋白,已报导拟南芥AtFRD3和水稻OsFRDL1能够促进木质部中柠檬酸–三价铁螯合物向地上部运输[18-20]。前期研究报道,细胞内自由态铁也可以和尼克酰胺(NA)螯合剂结合,将铁通过韧皮部从老叶运输到新叶,Fe-NA的转运主要依赖于YS/YSL家族成员[21]。另外,早期研究发现,拟南芥一个寡肽转运蛋白AtOPT3 (oligopeptide transporter 3)可能在维管束的铁转运过程中发挥着重要作用,但是其转运过程却不依赖NA[22],说明它转运的可能是多肽或多肽类分子螯合的铁。近期研究揭示,OPT3是韧皮部特异的铁转运蛋白,OPT3将铁加载到韧皮部中,促进铁从木质部到韧皮部的再循环,并调节地上部茎到地下部根的铁状态的系统信号传导和铁从成熟组织向发育组织的重新分配[23]。转运到特定器官或组织的铁还需要进一步分配到合适的细胞器中以便完成各种生命活动,三价铁还原酶FRO7和转运蛋白PIC1参与拟南芥中铁向叶绿体的跨膜转运[24-25]。水稻铁转运蛋白MIT将铁运输到线粒体[26]。NRAMP转运家族蛋白NRAMP3和NRAMP4负责将铁从液泡输出到细胞质,而VIT1则负责从细胞质向液泡中输入铁[27-28]。但是由于发育中的种子和母体并没有维管组织相连,铁是如何运输到种子里去的分子机制目前还不是很清楚[29]。
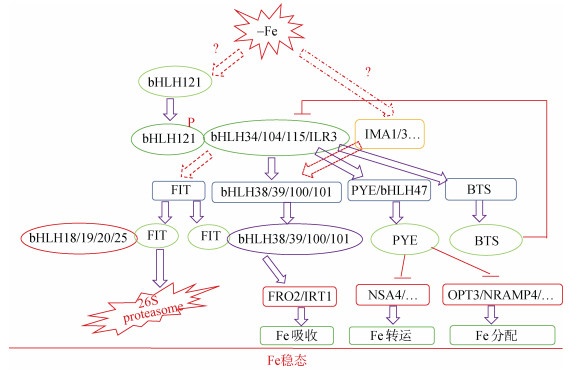
3 策略Ⅰ植物铁稳态的转录调控铁是植物所必需的微量元素,但由于铁活跃的化学性质,过多的铁将会产生大量的活性氧(reactive oxygen species, ROS),对细胞产生毒害,甚至死亡。而铁主要是由根系从土壤中直接吸收获得,因此调控铁的吸收对于保持细胞铁稳态至关重要。在拟南芥中,铁主要吸收相关基因是FRO2和IRT1,分别负责将Fe3+还原成Fe2+和将Fe2+从根际转运到根细胞内[3]。研究表明,拟南芥细胞内铁稳态主要在转录水平上受到多个来自bHLH(basic-helix-loop-helix) 家族的转录因子调控。其中FIT/bHLH29和ILR3 (IAA-LEUCINE RESISTANT3)/ bHLH105是该信号通路中两个关键节点[30-31]。FIT和转录因子bHLH38、bHLH39、bHLH100、bHLH101之一形成异源二聚体,调节IRT1和FRO2等基因的活性[32-35]。FIT和bHLH38、bHLH39、bHLH100、bHLH101之一形成的异源二聚体的活性可被转录因子bHLH18、bHLH19、bHLH20、bHLH25负调控,bHLH18、bHLH19、bHLH20、bHLH25一旦与FIT相互作用就会通过26S蛋白酶体途径促进FIT降解[36]。FIT的降解不仅受到26S蛋白酶的控制[37-38],而且受到乙烯信号传导途径中的转录因子EIN3和EIL1的调控[39]。ILR3及其3个相近的同源物(bHLH34、bHLH104和bHLH115)可以形成同源二聚体和异源二聚体,直接激活bHLH38、bHLH39、bHLH100、bHLH101和PYE/ bHLH47的表达,从正负两个方面来调节铁稳态[31, 40-41],这是因为PYE是参与细胞内铁稳态的负调控转录因子[42],而bHLH38、bHLH39、bHLH100、bHLH101是参与细胞内铁稳态的正调控转录因子。ILR3和bHLH115同E3连接酶BTS (brutus)互作后通过26S蛋白酶体途径而降解[43]。
FIT及其互作蛋白bHLH38、bHLH39、bHLH100和bHLH101直接调控铁吸收基因FRO2和IRT1的表达,但这些转录因子自身在转录水平上也受缺铁调控,暗示还有另外的转录因子调控着FIT及其互作蛋白基因的转录表达[3]。经过近5年的研究,现在已经明确了FIT互作蛋白基因转录调控的上游转录因子是ILR3及其3个相近的同源物bHLH34、bHLH104和bHLH115。但是这些上游转录因子并不能直接激活FIT的表达,也就是这些转录因子并不能和FIT的启动子结合。表明这些转录因子激活FIT的表达不是直接的而是间接的[31, 40-41],FIT和这些转录因子之间应该还有一个“桥梁因子”。最近,国内外3个独立课题组采用不同的技术手段都鉴定到这个“桥梁因子”就是转录因子bHLH121[44-46]。虽然这3篇独立研究报告某些结果略有不同,但总体上结论一致并且相互补充,更加完善了拟南芥铁稳态的分子调控网络。Kim等[45]首先构建了IRT1启动子连接报告基因LUC的转基因株系,并对该株系进行EMS(ethyl methanesulphonate)化学诱变,创建突变体库,然后通过筛选突变体库鉴定到bHLH121/URI(upstream regulator of IRT1),而另外两个课题组分别通过酵母单杂[46]和免疫共沉淀结合质谱分析以及酵母双杂[44]筛选获得。Kim等[45]并证明URI可以直接和bHLH38、bHLH39、bHLH100和bHLH101的启动子结合,但不能和FIT的启动子结合,该结果和Gao等[44]的结果一致;但Lei等[46]结果表明,虽然bHLH121自身对这些靶基因并没有激活或抑制活性,但bHLH121可以和FIT启动子上的E box元件直接结合。3篇研究都证明,bHLH121和bHLH IVc组的转录因子可以互作,形成异源二聚体,从而激活了FIT、bHLH38、bHLH39、bHLH100、bHLH101、PEY等基因的转录表达。Kim等[45]进一步证明缺铁诱导了bHLH121的磷酸化,磷酸化的bHLH121在缺铁条件下积累,激活了bHLH38、bHLH39、bHLH100、bHLH101、PEY、BTS和BTSL1的表达,进一步激活了FRO2和IRT1的表达,增加对铁的吸收;而在铁充足条件下,磷酸化的bHLH121通过26S蛋白酶体途径而降解,进而减少铁的吸收,维持胞内铁稳态。
4 小肽对策略Ⅰ植物铁稳态的调控维持体内铁稳态是植物生长发育的基础,而调控铁吸收转运是维持铁稳态的前提条件。除上述转录因子所构成的调控网络对铁稳态进行调控外,前期研究发现,缺铁诱导大量未知功能基因的上调表达,其中包括编码小肽IMA1及其同源基因。缺失IMA1及其他7个同源基因后,植物在正常铁条件下生长受阻,在土壤中如果不外源添加高浓度的铁,突变体存活不超过两周;相反,过表达IMA1后不仅增加了植株和种子铁含量,增加了对缺铁的耐受性,而且在铁充足的条件下,过表达植株的根系三价铁还原酶活性显著升高,缺铁诱导表达的铁吸收基因FRO2和IRT1都显著上调表达,表明过表达小肽IMA1在铁充足条件下激活了植株体内的缺铁响应机制,增加了对铁的吸收,进一步转录组分析发现,过表达IMA1植株在铁充足条件下,转录因子bHLH38、bHLH39出现显著上调表达[47],IMA1可能代表一条新的铁稳态调控机制[45]。
5 问题与展望铁是一切生物所必需的微量元素,过量或不足都会影响细胞的正常生理活动。因此精确调控铁的吸收转运,保持体内铁稳态是植物良好生长发育的基础,也是高产优质的前提条件,更是培育富铁农作物品种的理论基础。对于铁吸收转运和铁稳态这一重要的植物营养领域,国内外已进行了大量的研究并取得一系列研究成果。一些和铁吸收、转运等相关的基因已经成功克隆,功能得以验证;一些关键调控因子和信号分子已经被鉴定,极大地丰富了对植物响应缺铁机制的认识,逐步完善了铁稳态的分子调控网络(图 1)。总结国内外已经取得的研究成果并结合作者的思考,将该领域仍然存在的问题以及未来研究发展趋势概括为以下几个方面:①拟南芥为什么需要进化出如此多bHLH类转录因子进行铁吸收稳态的转录调控?是否所有或者绝大多数策略Ⅰ植物都具有这样的调控模式?未来随着越来越多植物基因组序列的测定,有望从进化角度思考策略Ⅰ植物铁吸收稳态的分子调控网络是否高度保守,以及何时发生变异的;②迄今,转录因子bHLH121是铁稳态分子网络最上游的转录调控因子,虽然其自身转录并不受缺铁诱导,但其翻译后磷酸化修饰却受到缺铁调控,目前其分子机制并不清楚,是哪一种蛋白激酶对其磷酸化的也不明确,未来对其研究必将进一步完善该分子调控网络;③虽然前期发现IMA1及其同源基因在拟南芥铁吸收和稳态方面发挥了重要的调控作用,并可能代表着一个新的调控模块[45],但是IMA1小肽是如何发挥其调控作用的仍然需要进一步探索。IMA1小肽基因在转录水平上受缺铁强烈诱导,但在蛋白水平却未能检测到IMA1的肽段[48],增加了对IMA1研究的难度;④植物是如何感知外界铁状态(不足、适宜、过多),进而及时地调控铁吸收基因的转录表达的机制还不清楚,也就是铁受体还没有得到公认。在水稻中,前期研究认为两个泛素化E3连接酶OsHRZ1和OsHRZ2 (haemerythrin motif-containing really interesting new gene (ring) and zinc-finger protein 1 and 2)是铁感应器(sensor),监测胞内铁状态[49];在拟南芥中,OsHRZ1和OsHRZ2的同源蛋白BTS (brutus)及其同源物BTSL1和BTSL2具有铁感应器的潜力[42-43, 50]。当然也有研究报道指出,IRT1具有双重功能,既可以转运铁也可以感受铁,也就是铁的转运受体(transceptor),在金属元素感知和信号传递方面起关键作用[51]。总之,目前无论是策略Ⅰ还是策略Ⅱ植物,铁的受体或者感应器还没有得到公认,这也是目前以及未来一段时间内植物铁营养领域最为关注的科学问题,也是最富挑战性的问题,一旦突破将对富铁作物培育具有巨大的理论和实践意义。
|
图 1 策略Ⅰ植物铁稳态调控分子网络 Fig. 1 Molecular regulatory network maintaining Fe homoeostasis in Strategy I plants |
| [1] |
Aung M S, Masuda H. Corrigendum: how does rice defend against excess iron?: Physiological and molecular mechanisms[J]. Frontiers in Plant Science, 2020, 11: 1102 DOI:10.3389/fpls.2020.01102 (  0) 0) |
| [2] |
Hänsch R, Mendel R R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl)[J]. Current Opinion in Plant Biology, 2009, 12(3): 259-266 DOI:10.1016/j.pbi.2009.05.006 (  0) 0) |
| [3] |
Kobayashi T, Nishizawa N K. Iron uptake, translocation, and regulation in higher plants[J]. Annual Review of Plant Biology, 2012, 63: 131-152 DOI:10.1146/annurev-arplant-042811-105522 (  0) 0) |
| [4] |
Li W F, Lan P. The understanding of the plant iron deficiency responses in strategy I plants and the role of ethylene in this process by omic approaches[J]. Frontiers in Plant Science, 2017, 8: 40 (  0) 0) |
| [5] |
Touraine B, Vignols F, Przybyla-Toscano J, et al. Iron-sulfur protein NFU2 is required for branched-chain amino acid synthesis in Arabidopsis roots[J]. Journal of Experimental Botany, 2019, 70(6): 1875-1889 DOI:10.1093/jxb/erz050 (  0) 0) |
| [6] |
Kim S A, Guerinot M L. Mining iron: Iron uptake and transport in plants[J]. FEBS Letters, 2007, 581(12): 2273-2280 DOI:10.1016/j.febslet.2007.04.043 (  0) 0) |
| [7] |
Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses[J]. Plant Physiology, 1986, 80(1): 175-180 DOI:10.1104/pp.80.1.175 (  0) 0) |
| [8] |
Nozoye T, Nagasaka S, Kobayashi T, et al. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants[J]. Journal of Biological Chemistry, 2011, 286(7): 5446-5454 DOI:10.1074/jbc.M110.180026 (  0) 0) |
| [9] |
Curie C, Panaviene Z, Loulergue C, et al. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake[J]. Nature, 2001, 409(6818): 346-349 DOI:10.1038/35053080 (  0) 0) |
| [10] |
Santi S, Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots[J]. The New Phytologist, 2009, 183(4): 1072-1084 DOI:10.1111/j.1469-8137.2009.02908.x (  0) 0) |
| [11] |
Fourcroy P, Tissot N, Gaymard F, et al. Facilitated Fe nutrition by phenolic compounds excreted by the Arabidopsis ABCG37/PDR9 transporter requires the IRT1/FRO2 high-affinity root Fe(2+) transport system[J]. Molecular Plant, 2016, 9(3): 485-488 DOI:10.1016/j.molp.2015.09.010 (  0) 0) |
| [12] |
Robe K, Conejero G, Gao F, et al. Coumarin accumulation and trafficking in Arabidopsis thaliana: A complex and dynamic process[J]. The New Phytologist, 2021, 229(4): 2062-2079 DOI:10.1111/nph.17090 (  0) 0) |
| [13] |
Robe K, Izquierdo E, Vignols F, et al. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health[J]. Trends in Plant Science, 2021, 26(3): 248-259 DOI:10.1016/j.tplants.2020.10.008 (  0) 0) |
| [14] |
Tsai H H, Rodríguez-Celma J, Lan P, et al. Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization[J]. Plant Physiology, 2018, 177(1): 194-207 DOI:10.1104/pp.18.00178 (  0) 0) |
| [15] |
Eide D, Broderius M, Fett J, et al. A novel iron-regulated metal transporter from plants identified by functional expression in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(11): 5624-5628 DOI:10.1073/pnas.93.11.5624 (  0) 0) |
| [16] |
Robinson N J, Procter C M, Connolly E L, et al. A ferric-chelate reductase for iron uptake from soils[J]. Nature, 1999, 397(6721): 694-697 DOI:10.1038/17800 (  0) 0) |
| [17] |
Fourcroy P, Sisó-Terraza P, Sudre D, et al. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency[J]. The New Phytologist, 2014, 201(1): 155-167 DOI:10.1111/nph.12471 (  0) 0) |
| [18] |
Durrett T P, Gassmann W, Rogers E E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation[J]. Plant Physiology, 2007, 144(1): 197-205 DOI:10.1104/pp.107.097162 (  0) 0) |
| [19] |
Green L S, Rogers E E. FRD3 controls iron localization in Arabidopsis[J]. Plant Physiology, 2004, 136(1): 2523-2531 DOI:10.1104/pp.104.045633 (  0) 0) |
| [20] |
Yokosho K, Yamaji N, Ueno D, et al. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice[J]. Plant Physiology, 2009, 149(1): 297-305 DOI:10.1104/pp.108.128132 (  0) 0) |
| [21] |
Curie C, Cassin G, Couch D, et al. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters[J]. Annals of Botany, 2009, 103(1): 1-11 DOI:10.1093/aob/mcn207 (  0) 0) |
| [22] |
Stacey M G, Patel A, McClain W E, et al. The Arabidopsis AtOPT3 protein functions in metal homeostasis and movement of iron to developing seeds[J]. Plant Physiology, 2008, 146(2): 323-324 (  0) 0) |
| [23] |
Zhai Z Y, Gayomba S R, Jung H I, et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis[J]. The Plant Cell, 2014, 26(5): 2249-2264 DOI:10.1105/tpc.114.123737 (  0) 0) |
| [24] |
Duy D, Stübe R, Wanner G, et al. The chloroplast permease PIC1 regulates plant growth and development by directing homeostasis and transport of iron[J]. Plant Physiology, 2011, 155(4): 1709-1722 DOI:10.1104/pp.110.170233 (  0) 0) |
| [25] |
Jeong J, Cohu C, Kerkeb L, et al. Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions[J]. PNAS, 2008, 105(30): 10619-10624 DOI:10.1073/pnas.0708367105 (  0) 0) |
| [26] |
Bashir K, Ishimaru Y, Shimo H, et al. The rice mitochondrial iron transporter is essential for plant growth[J]. Nature Communications, 2011, 2: 322 DOI:10.1038/ncomms1326 (  0) 0) |
| [27] |
Kim S A, Punshon T, Lanzirotti A, et al. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1[J]. Science, 2006, 314(5803): 1295-1298 DOI:10.1126/science.1132563 (  0) 0) |
| [28] |
Lanquar V, Lelièvre F, Bolte S, et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron[J]. The EMBO Journal, 2005, 24(23): 4041-4051 DOI:10.1038/sj.emboj.7600864 (  0) 0) |
| [29] |
Mari S, Bailly C, Thomine S. Handing off iron to the next generation: How does it get into seeds and what for?[J]. The Biochemical Journal, 2020, 477(1): 259-274 DOI:10.1042/BCJ20190188 (  0) 0) |
| [30] |
Tissot N, Robe K, Gao F, et al. Transcriptional integration of the responses to iron availability in Arabidopsis by the bHLH factor ILR3[J]. The New Phytologist, 2019, 223(3): 1433-1446 DOI:10.1111/nph.15753 (  0) 0) |
| [31] |
Zhang J, Liu B, Li M S, et al. The bHLH transcription factor bHLH104 interacts with IAA-LEUCINE RESISTANT3 and modulates iron homeostasis in Arabidopsis[J]. The Plant Cell, 2015, 27(3): 787-805 DOI:10.1105/tpc.114.132704 (  0) 0) |
| [32] |
Colangelo E P, Guerinot M L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response[J]. The Plant Cell, 2004, 16(12): 3400-3412 DOI:10.1105/tpc.104.024315 (  0) 0) |
| [33] |
Sivitz A B, Hermand V, Curie C, et al. Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway[J]. PLoS One, 2012, 7(9): e44843 DOI:10.1371/journal.pone.0044843 (  0) 0) |
| [34] |
Wang N, Cui Y, Liu Y, et al. Requirement and functional redundancy of ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana[J]. Molecular Plant, 2013, 6(2): 503-513 DOI:10.1093/mp/sss089 (  0) 0) |
| [35] |
Yuan Y X, Wu H L, Wang N, et al. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis[J]. Cell Research, 2008, 18(3): 385-397 DOI:10.1038/cr.2008.26 (  0) 0) |
| [36] |
Cui Y, Chen C L, Cui M, et al. Four IVa bHLH transcription factors are novel interactors of FIT and mediate JA inhibition of iron uptake in Arabidopsis[J]. Molecular Plant, 2018, 11(9): 1166-1183 DOI:10.1016/j.molp.2018.06.005 (  0) 0) |
| [37] |
Rodríguez-Celma J, Connorton J M, Kruse I, et al. Arabidopsis BRUTUS-like E3 ligases negatively regulate iron uptake by targeting transcription factor FIT for recycling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(35): 17584-17591 DOI:10.1073/pnas.1907971116 (  0) 0) |
| [38] |
Sivitz A, Grinvalds C, Barberon M, et al. Proteasome-mediated turnover of the transcriptional activator FIT is required for plant iron-deficiency responses[J]. The Plant Journal, 2011, 66(6): 1044-1052 DOI:10.1111/j.1365-313X.2011.04565.x (  0) 0) |
| [39] |
Lingam S, Mohrbacher J, Brumbarova T, et al. Interaction between the bHLH transcription factor FIT and ETHYLENE INSENSITIVE3/ETHYLENE INSENSITIVE3-LIKE1 reveals molecular linkage between the regulation of iron acquisition and ethylene signaling in Arabidopsis[J]. The Plant Cell, 2011, 23(5): 1815-1829 (  0) 0) |
| [40] |
Li X L, Zhang H M, Ai Q, et al. Two bHLH transcription factors, bHLH34 and bHLH104, regulate iron homeostasis in Arabidopsis thaliana[J]. Plant Physiology, 2016, 170(4): 2478-2493 (  0) 0) |
| [41] |
Liang G, Zhang H M, Li X L, et al. bHLH transcription factor bHLH115 regulates iron homeostasis in Arabidopsis thaliana[J]. Journal of Experimental Botany, 2017, 68(7): 1743-1755 (  0) 0) |
| [42] |
Long T A, Tsukagoshi H, Busch W, et al. The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots[J]. The Plant Cell, 2010, 22(7): 2219-2236 (  0) 0) |
| [43] |
Selote D, Samira R, Matthiadis A, et al. Iron-binding E3 ligase mediates iron response in plants by targeting basic helix-loop-helix transcription factors[J]. Plant Physiology, 2015, 167(1): 273-286 (  0) 0) |
| [44] |
Gao F, Robe K, Bettembourg M, et al. The transcription factor bHLH121 interacts with bHLH105(ILR3) and its closest homologs to regulate iron homeostasis in Arabidopsis[J]. The Plant Cell, 2020, 32(2): 508-524 (  0) 0) |
| [45] |
Kim S A, LaCroix I S, Gerber S A, et al. The iron deficiency response in Arabidopsis thaliana requires the phosphorylated transcription factor URI[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(50): 24933-24942 (  0) 0) |
| [46] |
Lei R H, Li Y, Cai Y R, et al. bHLH121 functions as a direct link that facilitates the activation of FIT by bHLH IVc transcription factors for maintaining Fe homeostasis in Arabidopsis[J]. Molecular Plant, 2020, 13(4): 634-649 (  0) 0) |
| [47] |
Grillet L, Lan P, Li W F, et al. IRON MAN is a ubiquitous family of peptides that control iron transport in plants[J]. Nature Plants, 2018, 4(11): 953-963 (  0) 0) |
| [48] |
Lan P, Li W F, Wen T N, et al. iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis[J]. Plant Physiology, 2011, 155(2): 821-834 (  0) 0) |
| [49] |
Kobayashi T, Nagasaka S, Senoura T, et al. Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation[J]. Nature Communications, 2013, 4: 2792 (  0) 0) |
| [50] |
Rodríguez-Celma J, Chou H, Kobayashi T, et al. Hemerythrin E3 ubiquitin ligases as negative regulators of iron homeostasis in plants[J]. Frontiers in Plant Science, 2019, 10: 98 (  0) 0) |
| [51] |
Cointry V, Vert G. The bifunctional transporter-receptor IRT1 at the heart of metal sensing and signalling[J]. The New Phytologist, 2019, 223(3): 1173-1178 (  0) 0) |
2. State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China
 2021, Vol. 53
2021, Vol. 53


