2020年我国农药利用率达40.6%,相比世界发达农业国家,国内粮食生产平均每公顷使用农药量仍然偏高[1]。除草剂和杀虫剂在农药市场的销售份额也在不断提升,目前杀虫剂市场上新烟碱类、菊酯类和有机磷类的老品种占据着销售前三甲[2]。自20世纪90年代,新烟碱类杀虫剂引进以来,其已成为世界上使用最广泛的一类杀虫剂,已在120个国家注册[3-4]。2019年数据表明,以吡虫啉为有效成分的产品登记数量最多,合计1 393种,其次为啶虫脒731种,噻虫嗪518种,有效成分登记平均用量排序为啶虫脒、吡虫啉、烯啶虫胺[5]。新烟碱类杀虫剂不仅销量和使用量占比较大,其环境残留问题也日趋严峻,在土壤及底泥、水体、非靶标植物中均已发现其残留[6-8]。研究表明,新烟碱类杀虫剂在土壤中可残留长达数月甚至数年的时间[9]。在我国小麦产区土壤中氯氟氰菊酯、吡虫啉和氯氰菊酯的平均残留量最高,分别为44.12、27.08、23.31 μg/kg。农药在进入环境后还发生代谢过程。在我国水稻、玉米和小麦粮食产区土壤样品中,吡虫啉、啶虫脒和噻虫嗪的5种代谢物的检出率均为100%,其在土壤样品中的总残留水平是3个母体总和的2.4倍(均值)[10]。新烟碱类化合物对昆虫有明显的毒性,对哺乳动物、鸟类和其他高等生物的毒性较低。然而,最近的体外、体内和生态领域研究表明,新烟碱类杀虫剂可对脊椎动物和无脊椎动物以及哺乳动物产生不利影响[11]。
1 新烟碱类杀虫剂的结构与理化性质新烟碱类杀虫剂,通过与昆虫中枢神经系统乙酰胆碱酯酶受体蛋白结合而起拮抗作用[12]。新烟碱类杀虫剂一般由5部分组成,分别为杂环基团、桥链部分、功能基团、正电中心、取代基部分[13]。根据功能基团的不同可分为3类[14]:N-硝基胍类,如吡虫啉、噻虫胺、噻虫嗪和呋虫胺;硝基亚甲基类,如烯啶虫胺;N-氰基脒类,如啶虫脒和噻虫啉。根据取代基部分的不同可分为2类:链状新烟碱类杀虫剂,即取代基部分为脂肪链状结构,如呋虫胺;环状新烟碱类杀虫剂,即取代基部分与正电中心形成杂环,如噻虫嗪。常见的新烟碱类杀虫剂的具体结构与理化性质见表 1。
|
|
表 1 新烟碱类杀虫剂理化性质 Table 1 Physical and chemical properties of neonicotinoid insecticides |
农药化学降解是指农药在环境中受到一些物理化学因素(如光、热、化学物质)的影响而发生降解,主要包括光降解和水解[15-17]。
2.1 光降解光降解过程通常包含了农药内部C–C、C–H、C–O、C–N化学键断裂、异构化、分子内重排或分子间反应等过程,造成有机污染物分子结构的不可逆改变,因此光降解是有机污染物在环境中比较彻底的降解途径[18-19]。由于新烟碱类杀虫剂的光降解只能在土壤表层发生,所以一般研究液体介质中的光降解,因此影响光降解行为的主要因素包括初始浓度、光源、不同水体、有机溶剂、pH等[20-21]。初始浓度越高,新烟碱类杀虫剂的光解速率越慢,半衰期越长。初始浓度为5.0、10.0和20.0 mg/L的啶虫脒在水中的半衰期分别为60.26、69.30和92.40 min。Mahapatra等[22]发现,紫外灯照射下吡虫啉的光解半衰期13.6 h比自然光照下的16.1 h要快。啶虫脒在太阳光下的光解半衰期为147.48 h,在高压汞灯和紫外灯下的光解半衰期分别为69.30 min和48.13 min;啶虫脒在4种不同水质中的光解速率有明显差异,光解速率从快到慢依次为:重蒸水 > 自来水 > 巢湖水 > 稻田水[23]。在紫外光的照射下,呋虫胺在4种有机溶剂中的光解反应快慢顺序为:乙酸乙酯 > 甲醇 > 乙醇 > 丙酮,光解半衰期分别为3.39、4.83、5.05和9.23 h,在不同的pH条件下,当pH由酸性变为碱性时,呋虫胺的光解速率逐渐加快,其在pH为5、7、8和9的条件下的光解半衰期分别为12.42、12.06、10.84和8.45 h[24]。新烟碱类杀虫剂光化学反应主要类型有光氧化、光水解、光敏化作用。表 2列举了几种新烟碱类杀虫剂的光降解途径及产物。
|
|
表 2 新烟碱类杀虫剂光降解途径 Table 2 Photodegradation pathways of neonicotinoid insecticides |
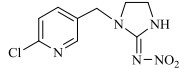
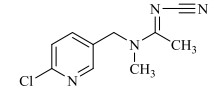
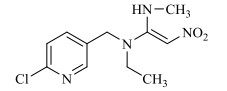
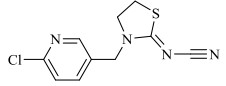
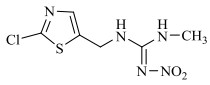
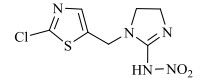
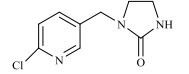
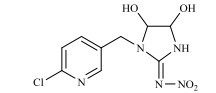
农药的水解反应是农药分子与水分子发生相互作用的化学反应过程,其反应本质是水分子的亲核基团(H2O或OH–)进攻农药分子的亲电基团(C、N、S、P等),取代离去基团(Cl-、苯酚盐等),属于亲核取代反应[31]。新烟碱类杀虫剂的水解和温度呈正相关,噻虫啉在25℃和pH 9时,水解半衰期为59.8 d,当温度升至50℃,水解速率增加,半衰期缩减到39.8 d。一定温度下,新烟碱类杀虫剂的水解受pH的影响,在酸性和中性条件下难以降解,一般属于碱性水解,随着pH的增加,水解速率不断加快,但是二价金属离子(Cu2+,Ni2+,Zn2+)和矿物(高岭石、针铁矿、TiO2)对水解速率无影响[32]。如图 1所示,噻虫嗪的水解过程主要包括C=N双键被OH–攻击,生成C=O键、C–N键断裂、环羟基化以及脱氯[29, 33-35];呋虫胺的水解过程主要为OH–作为亲核基团进攻亲电基团(C+=N),并发生取代反应生成C=O键[24];噻虫胺的水解过程主要涉及羟基自由基进攻N=C生成C=O键的过程以及C–N键的断裂[30];由于吡虫啉咪唑烷上的NO2是强吸电子基团,它使C=N位上的C原子易受OH–进攻发生反应[36],吡虫啉的水解过程主要涉及羟基自由基进攻N+=C生成C=O键的过程以及C–N键的断裂。马畅[37]研究发现,H2O分子的OH–进攻氯噻啉分子中与咪唑啉环相连的N–N单键,形成N–H键,生成M216和HNO3小分子,H2O分子的OH–进攻M216分子中与噻唑环上的C–Cl单键,形成C–O键,生成M198和HCl小分子。

|
图 1 典型新烟碱类杀虫剂的水解机理 Fig. 1 Hydrolysis mechanisms of three representative neonicotinoids |
生物修复技术是去除环境污染物的有效且经济实用的方案。土壤中含有大量的藻类、细菌、真菌、无脊椎动物和原生动物。土壤微生物种群在植物生长调控、植物病虫害防治、土壤结构维持、养分循环利用和污染物转化等方面发挥着重要作用[38-39]。利用覆盖地球一半生物量的微生物是最好的生物修复工具,因为其可以在很短的时间内大规模地生长和繁殖,且选择性强,可以采用原位修复,不易产生抗性,具有良好的成本效益[40-43]。
3.1 降解菌降解新烟碱类杀虫剂的微生物包括芽孢杆菌、假单胞菌、根瘤菌、贪噬菌、放线菌和白腐真菌,已被分离和鉴定[44],如表 3所示。这些微生物可以在实验室和野外条件下降解新烟碱类杀虫剂。
|
|
表 3 分离微生物降解新烟碱类杀虫剂的研究 Table 3 Degradation studies of neonicotinoid insecticides by isolated microorganisms |
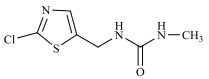
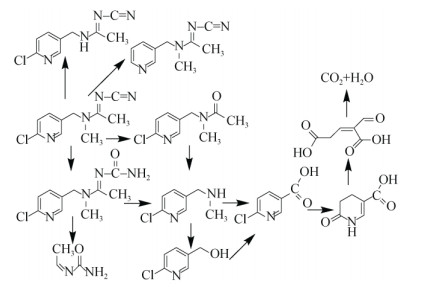
吡虫啉降解微生物已报道的代谢途径如图 2所示。羟基化和硝基还原是吡虫啉的两种主要的微生物降解途径[60-62]。羟基化途径:吡虫啉(IMI)生成5-Hydroxy IMI,其在脱水酶作用下脱水生成Olefin IMI,Olefin IMI含有不饱和双键,更容易发生降解,最终分解成CO2;硝基还原途径:吡虫啉(IMI)生成亚硝基(nitroso)、胍基(guanidine)和羰基(urea)IMI。两种途径都会产生6-氯烟酸和6-羟基烟酸,6-氯烟酸为易分解有机物,氧化后产生H2O和CO2。啶虫脒的微生物代谢途径如图 3所示。一般情况下,啶虫脒的C≡N被氧化断裂生成N-酰胺衍生物,其经过不对称裂解,产生两种中间产物,中间产物之一快速生成6-氯烟酸[50.63],最终矿化为H2O和CO2。Yang等[64]发现,啶虫脒存在不产生中间产物的两种降解途径,即直接脱氯、脱甲基得到最终产物;微生物系统不能将此最终产物转化为其他产物,但在动植物系统中发现其可继续降解[65]。如图 4所示,微生物可以通过多种途径降解噻虫嗪,硝基还原代谢途径与吡虫啉类似,生成亚硝基(nitroso)、胍基(guanidine)和羰基(urea)[66];类似于啶虫脒的生物降解途径,即噻虫嗪通过去甲基化途径转化为去甲基–噻虫嗪;恶二嗪环的裂解[67-68]途径。如图 5所示,噻虫胺微生物降解途径主要有3种:一种是C–N键断裂,生成两种产物;一种是先脱氮再脱氯,部分硝基胍中N–N键的断裂和噻唑基中C–Cl键的断裂[54];另一种是硝基亚氨基部分转化为尿素化合物[69-70]。

|
图 2 吡虫啉的微生物降解途径 Fig. 2 Microbial degradation pathways of imidacloprid |
|
图 3 啶虫脒的微生物降解途径 Fig. 3 Microbial degradation pathways of acetamiprid |

|
图 4 噻虫嗪的微生物降解途径 Fig. 4 Microbial degradation pathways of thiamethoxam |

|
图 5 噻虫胺的微生物降解途径 Fig. 5 Microbial degradation pathways of clothianidin |
农药在农业生产中使用广泛,以提高粮食产量,满足日益增长的人口需求。随着生产力的提高,化学农药的使用导致了一些环境问题,如土壤肥力和生物多样性降低,土壤酸化程度增加,生物的抗药性增强等。这些化学品污染空气、地面和水体[71],若在环境中停留时间较长,也会进入食物链并影响整个生态系统。微生物修复是一种有效的净化污染的技术。通过加强自然生物降解过程,可使微生物迅速适应变化和有毒的环境,实现对污染物的降解,但是目前对于微生物的作用机制研究还不够深入,全面了解微生物群落及其对自然环境和污染物的响应,对于发展生态稳定、新颖和潜在的生物修复方法非常重要。因此,针对未来研究的重点提出以下建议:①探究降解产物的毒性,并研究降解产物的毒理机制;②考虑极端微生物在生物修复中的惊人作用,需要进行更深入的研究,以便识别新的物种,并研究其在极端环境中作用的机制;③生物体的生物降解潜力是由单个细胞内的遗传成分决定的[72],为了了解其在污染环境中的降解机制,对其功能基因和酶的研究十分重要,因为这些基因可以与其他生物体结合。具有更强降解污染物能力的转基因微生物在这一领域具有重要的研究前景。
| [1] |
2021年农药行业市场发展趋势[J]. 农村新技术, 2021(3): 46–47.
(  0) 0) |
| [2] |
芦志成, 李慧超, 关爱莹, 等. 2015—2019年除草剂和杀虫(螨)剂创制品种概述[J]. 农药, 2020, 59(2): 79-90 DOI:10.16820/j.cnki.1006-0413.2020.02.001 (  0) 0) |
| [3] |
Bartlett A J, Hedges A M, Intini K D, et al. Acute and chronic toxicity of neonicotinoid and butenolide insecticides to the freshwater amphipod, Hyalella azteca[J]. Ecotoxicology and Environmental Safety, 2019, 175: 215-223 DOI:10.1016/j.ecoenv.2019.03.038 (  0) 0) |
| [4] |
Li Z G, Yu T T, Chen Y P, et al. Brain transcriptome of honey bees (Apis mellifera) exhibiting impaired olfactory learning induced by a sublethal dose of imidacloprid[J]. Pesticide Biochemistry and Physiology, 2019, 156: 36-43 DOI:10.1016/j.pestbp.2019.02.001 (  0) 0) |
| [5] |
毛连纲, 徐冬梅, 袁善奎, 等. 基于推荐用量分析我国新烟碱类杀虫剂的登记现状[J]. 植物保护, 2020, 46(5): 200-210 DOI:10.16688/j.zwbh.2019297 (  0) 0) |
| [6] |
Anderson J C, Dubetz C, Palace V P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects[J]. Science of the Total Environment, 2015, 505: 409-422 DOI:10.1016/j.scitotenv.2014.09.090 (  0) 0) |
| [7] |
Botías C, David A, Horwood J, et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees[J]. Environmental Science & Technology, 2015, 49(21): 12731-12740 (  0) 0) |
| [8] |
Xue Y G, Limay-Rios V, Smith J, et al. Quantifying neonicotinoid insecticide residues escaping during maize planting with vacuum planters[J]. Environmental Science & Technology, 2015, 49(21): 13003-13011 (  0) 0) |
| [9] |
Bonmatin J M, Giorio C, Girolami V, et al. Environmental fate and exposure; neonicotinoids and fipronil[J]. Environmental Science and Pollution Research International, 2015, 22(1): 35-67 DOI:10.1007/s11356-014-3332-7 (  0) 0) |
| [10] |
姜朵朵. 典型农药在我国三种粮食产地残留特征及膳食风险评估[D]. 北京: 中国农业科学院, 2021.
(  0) 0) |
| [11] |
Chrétien F, Giroux I, Thériault G, et al. Surface runoff and subsurface tile drain losses of neonicotinoids and companion herbicides at edge-of-field[J]. Environmental Pollution, 2017, 224: 255-264 DOI:10.1016/j.envpol.2017.02.002 (  0) 0) |
| [12] |
le Questel J Y, Graton J, Cerón-Carrasco J P, et al. New insights on the molecular features and electrophysiological properties of dinotefuran, imidacloprid and acetamiprid neonicotinoid insecticides[J]. Bioorganic & Medicinal Chemistry, 2011, 19(24): 7623-7634 (  0) 0) |
| [13] |
魏立娜, 叶非. 新烟碱类杀虫剂的作用机制、应用及结构改造的研究进展[J]. 农药科学与管理, 2013, 34(5): 27-34 DOI:10.3969/j.issn.1002-5480.2013.05.008 (  0) 0) |
| [14] |
Morrissey C A, Mineau P, Devries J H, et al. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review[J]. Environment International, 2015, 74: 291-303 (  0) 0) |
| [15] |
陈梦现, 欧晓明, 刘红玉, 等. 新农药吩胺霉素的光化学降解[J]. 农药, 2016, 55(9): 654-657 DOI:10.16820/j.cnki.1006-0413.2016.09.007 (  0) 0) |
| [16] |
Ngim K K, Crosby D G. Abiotic processes influencing fipronil and desthiofipronil dissipation in California, USA, rice fields[J]. Environmental Toxicology and Chemistry, 2001, 20(5): 972-977 DOI:10.1002/etc.5620200506 (  0) 0) |
| [17] |
戴魏, 刘双清, 李晓刚, 等. 新农药联苯肼酯的光化学降解[J]. 环境化学, 2014, 33(2): 334-340 (  0) 0) |
| [18] |
丁绍武, 张鹏. 新烟碱类杀虫剂吡虫啉的残留危害及降解特性分析[J]. 现代农业科技, 2019(19): 121-123 DOI:10.3969/j.issn.1007-5739.2019.19.078 (  0) 0) |
| [19] |
李敏, 赵会君, 屈欢, 等. 新烟碱类杀虫剂潜在环境风险及光降解行为研究进展[J]. 农药, 2019, 58(3): 170-173 DOI:10.16820/j.cnki.1006-0413.2019.03.003 (  0) 0) |
| [20] |
Hilton M J, Jarvis T D, Ricketts D C. The degradation rate of thiamethoxam in European field studies[J]. Pest Management Science, 2016, 72(2): 388-397 DOI:10.1002/ps.4024 (  0) 0) |
| [21] |
李田田, 郑珊珊, 王晶, 等. 新烟碱类农药的污染现状及转化行为研究进展[J]. 生态毒理学报, 2018, 13(4): 9-21 (  0) 0) |
| [22] |
Mahapatra B, Adak T, Patil N K B, et al. Effect of abiotic factors on degradation of imidacloprid[J]. Bulletin of Environmental Contamination and Toxicology, 2017, 99(4): 475-480 (  0) 0) |
| [23] |
翟伟. 啶虫脒的光化学降解研究[D]. 安徽农业大学, 2008.
(  0) 0) |
| [24] |
张磊磊. 呋虫胺的水解和光化学降解研究[D]. 新乡: 河南师范大学, 2014.
(  0) 0) |
| [25] |
Wamhoff H, Schneider V. Photodegradation of imidacloprid[J]. Journal of Agricultural and Food Chemistry, 1999, 47(4): 1730-1734 (  0) 0) |
| [26] |
Moza P N, Hustert K, Feicht E, et al. Photolysis of imidacloprid in aqueous solution[J]. Chemosphere, 1998, 36(3): 497-502 (  0) 0) |
| [27] |
Malato S, Caceres J, Agüera A, et al. Degradation of imidacloprid in water by photo-Fenton and TiO2 photocatalysis at a solar pilot plant: A comparative study[J]. Environmental Science & Technology, 2001, 35(21): 4359-4366 (  0) 0) |
| [28] |
葛峰, 单正军, 戴亦军, 等. 烯式吡虫啉(olefin IMI)光解及其光解产物研究[J]. 生态与农村环境学报, 2009, 25(2): 103-106 (  0) 0) |
| [29] |
郑立庆. 噻虫嗪的水解与光解作用及对土壤呼吸作用影响研究[D]. 哈尔滨: 哈尔滨工业大学, 2006.
(  0) 0) |
| [30] |
刘保东. 噻虫胺的水解和光化学降解研究[D]. 新乡: 河南师范大学, 2013.
(  0) 0) |
| [31] |
王敏欣, 朱书全. 水环境中农药的水解研究分析[J]. 黑龙江科技学院学报, 2004, 14(2): 74-77 (  0) 0) |
| [32] |
Todey S A, Fallon A M, Arnold W A. Neonicotinoid insecticide hydrolysis and photolysis: Rates and residual toxicity[J]. Environmental Toxicology and Chemistry, 2018, 37(11): 2797-2809 DOI:10.1002/etc.4256 (  0) 0) |
| [33] |
Nelson P N. A DFT mechanistic study of two possible hydrolytic evolution pathways of thiamethoxam; implications in food and environmental safety[J]. Computational and Theoretical Chemistry, 2021, 1202: 113333 DOI:10.1016/j.comptc.2021.113333 (  0) 0) |
| [34] | |
| [35] |
Karmakar R, Singh S B, Kulshrestha G. Kinetics and mechanism of the hydrolysis of thiamethoxam[J]. Journal of Environmental Science and Health Part B, Pesticides, Food Contaminants, and Agricultural Wastes, 2009, 44(5): 435-441 (  0) 0) |
| [36] |
郑巍, 宣日成, 刘维屏. 新农药吡虫啉水解动力学和机理研究[J]. 环境科学学报, 1999, 19(1): 101-104 DOI:10.3321/j.issn:0253-2468.1999.01.021 (  0) 0) |
| [37] |
马畅. 土壤和水环境中氯噻啉的降解行为和降解产物研究[D]. 北京: 中国农业科学院, 2019.
(  0) 0) |
| [38] |
Ajar N Y, Rajesh K, Sunil K, et al. Beneficial microbiomes: Biodiversity and potential biotechnological applications for sustainable agriculture and human health[J]. Journal of Applied Biology & Biotechnology, 2017, 5(6): 50607 (  0) 0) |
| [39] |
Aniefiok I E, Udo I J. Role of plants and microbes in bioremediation of petroleum hydrocarbons contaminated soils[J]. International Journal of Environmental Bioremediation & Biodegradation, 2019, 7(1): 1-19 (  0) 0) |
| [40] |
Bonmatin J M, Noome D A, Moreno H, et al. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize[J]. Environmental Pollution, 2019, 249: 949-958 DOI:10.1016/j.envpol.2019.03.099 (  0) 0) |
| [41] |
Bhatt P, Bhatt K, Huang Y H, et al. Esterase is a powerful tool for the biodegradation of pyrethroid insecticides[J]. Chemosphere, 2020, 244: 125507 DOI:10.1016/j.chemosphere.2019.125507 (  0) 0) |
| [42] |
Lin Z Q, Zhang W P, Pang S M, et al. Current approaches to and future perspectives on methomyl degradation in contaminated soil/water environments[J]. Molecules (Basel, Switzerland), 2020, 25(3): 738 DOI:10.3390/molecules25030738 (  0) 0) |
| [43] |
Zhang W P, Linz Q, Pang S M, et al. Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system[J]. Frontiers in Microbiology, 2020, 11: 00522 DOI:10.3389/fmicb.2020.00522 (  0) 0) |
| [44] |
Pang S M, Lin Z Q, Zhang Y M, et al. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches[J]. Toxics, 2020, 8(3): 65 DOI:10.3390/toxics8030065 (  0) 0) |
| [45] |
Guo L L, Dai Z L, Guo J J, et al. Oligotrophic bacterium Hymenobacter latericoloratus CGMCC 16346 degrades the neonicotinoid imidacloprid in surface water[J]. AMB Express, 2020, 10(1): 7 DOI:10.1186/s13568-019-0942-y (  0) 0) |
| [46] |
Wu P, Zhang X W, Niu T, et al. The imidacloprid remediation, soil fertility enhancement and microbial community change in soil by Rhodopseudomonas Capsulata using effluent as carbon source[J]. Environmental Pollution, 2020, 267: 114254 DOI:10.1016/j.envpol.2020.114254 (  0) 0) |
| [47] |
Mohammed Y M M, Badawy M E I. Biodegradation of imidacloprid in liquid media by an isolated wastewater fungus Aspergillus terreus YESM3[J]. Journal of Environmental Science and Health, Part B, 2017, 52(10): 752-761 DOI:10.1080/03601234.2017.1356666 (  0) 0) |
| [48] |
Gupta M, Mathur S, Sharma T K, et al. A study on metabolic prowess of Pseudomonas sp. RPT 52 to degrade imidacloprid, endosulfan and coragen[J]. Journal of Hazardous Materials, 2016, 301: 250-258 DOI:10.1016/j.jhazmat.2015.08.055 (  0) 0) |
| [49] |
Guo L, Fang W W, Guo L L, et al. Biodegradation of the neonicotinoid insecticide acetamiprid by actinomycetes Streptomyces canus CGMCC 13662 and characterization of the novel nitrile hydratase involved[J]. J Agric Food Chem, 2019, 67(21): 5922-5931 DOI:10.1021/acs.jafc.8b06513 (  0) 0) |
| [50] |
Sun S L, Fan Z X, Zhao Y X, et al. A novel nutrient deprivation-induced neonicotinoid insecticide acetamiprid degradation by Ensifer adhaerens CGMCC 6315[J]. Journal of Agricultural and Food Chemistry, 2019, 67(1): 63-71 DOI:10.1021/acs.jafc.8b06154 (  0) 0) |
| [51] |
Sun S L, Yang W L, Guo J J, et al. Biodegradation of the neonicotinoid insecticide acetamiprid in surface water by the bacterium Variovorax boronicumulans CGMCC 4969 and its enzymatic mechanism[J]. RSC Advances, 2017, 7(41): 25387-25397 DOI:10.1039/C7RA01501A (  0) 0) |
| [52] |
Shi Z K, Dong W L, Xin F X, et al. Characteristics and metabolic pathway of acetamiprid biodegradation by Fusarium sp. strain CS-3 isolated from soil[J]. Biodegradation, 2018, 29(6): 593-603 DOI:10.1007/s10532-018-9855-8 (  0) 0) |
| [53] |
Zhao Y X, Jiang H Y, Cheng X, et al. Neonicotinoid thiacloprid transformation by the N2-fixing bacterium Microvirga flocculans CGMCC 1.16731 and toxicity of the amide metabolite[J]. International Biodeterioration & Biodegradation, 2019, 145: 104806 (  0) 0) |
| [54] |
Rana S, Jindal V, Mandal K, et al. Thiamethoxam degradation by Pseudomonas and Bacillus strains isolated from agricultural soils[J]. Environmental Monitoring and Assessment, 2015, 187(5): 300 DOI:10.1007/s10661-015-4532-4 (  0) 0) |
| [55] |
Parte S G, Kharat A S. Aerobic degradation of clothianidin to 2-chloro-methyl thiazole and methyl 3-(thiazole-yl) methyl guanidine produced by Pseudomonas stutzeri smk[J]. Journal of Environmental and Public Health, 2019, 2019: 4807913 (  0) 0) |
| [56] |
Mori T, Wang J Q, Tanaka Y, et al. Bioremediation of the neonicotinoid insecticide clothianidin by the white-rot fungus Phanerochaete sordida[J]. Journal of Hazardous Materials, 2017, 321: 586-590 DOI:10.1016/j.jhazmat.2016.09.049 (  0) 0) |
| [57] |
Wang J Q, Tanaka Y, Ohno H, et al. Biotransformation and detoxification of the neonicotinoid insecticides nitenpyram and dinotefuran by Phanerochaete sordida YK-624[J]. Environmental Pollution, 2019, 252: 856-862 DOI:10.1016/j.envpol.2019.06.022 (  0) 0) |
| [58] |
Zhou L Y, Zhang L J, Sun S L, et al. Degradation of the neonicotinoid insecticide acetamiprid via the N-carbamoylimine derivate (IM-1-2) mediated by the nitrile hydratase of the nitrogen-fixing bacterium Ensifer meliloti CGMCC 7333[J]. Journal of Agricultural and Food Chemistry, 2014, 62(41): 9957-9964 DOI:10.1021/jf503557t (  0) 0) |
| [59] |
Ge F, Zhou L Y, Wang Y, et al. Hydrolysis of the neonicotinoid insecticide thiacloprid by the N2-fixing bacterium Ensifer meliloti CGMCC 7333[J]. International Biodeterioration & Biodegradation, 2014, 93: 10-17 (  0) 0) |
| [60] |
Fusetto R, Denecke S, Perry T, et al. Partitioning the roles of CYP6G1 and gut microbes in the metabolism of the insecticide imidacloprid in Drosophila melanogaster[J]. Scientific Reports, 2017, 7: 11339 DOI:10.1038/s41598-017-09800-2 (  0) 0) |
| [61] |
Lu T Q, Mao S Y, Sun S L, et al. Regulation of hydroxylation and nitroreduction pathways during metabolism of the neonicotinoid insecticide imidacloprid by Pseudomonas putida[J]. Journal of Agricultural and Food Chemistry, 2016, 64(24): 4866-4875 DOI:10.1021/acs.jafc.6b01376 (  0) 0) |
| [62] |
Sabourmoghaddam N, Zakaria M P, Omar D. Evidence for the microbial degradation of imidacloprid in soils of Cameron Highlands[J]. Journal of the Saudi Society of Agricultural Sciences, 2015, 14(2): 182-188 DOI:10.1016/j.jssas.2014.03.002 (  0) 0) |
| [63] |
Phugare S S, Jadhav J P. Biodegradation of acetamiprid by isolated bacterial Strain Rhodococcussp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori)[J]. CLEAN - Soil, Air, Water, 2015, 43(2): 296-304 DOI:10.1002/clen.201200563 (  0) 0) |
| [64] |
Yang H X, Wang X, Zheng J, et al. Biodegradation of acetamiprid by Pigmentiphaga sp. D-2 and the degradation pathway[J]. International Biodeterioration & Biodegradation, 2013, 85: 95-102 (  0) 0) |
| [65] |
Brunet J L, Badiou A, Belzunces L P. In vivo metabolic fate of[14C]-acetamiprid in six biological compartments of the honeybee, Apis mellifera L[J]. Pest Management Science, 2005, 61(8): 742-748 DOI:10.1002/ps.1046 (  0) 0) |
| [66] |
Zhou G C, Wang Y, Zhai S, et al. Biodegradation of the neonicotinoid insecticide thiamethoxam by the nitrogen-fixing and plant-growth-promoting rhizobacterium Ensifer adhaerens strain TMX-23[J]. Applied Microbiology and Biotechnology, 2013, 97(9): 4065-4074 DOI:10.1007/s00253-012-4638-3 (  0) 0) |
| [67] |
Zhou G C, Wang Y, Ma Y, et al. The metabolism of neonicotinoid insecticide thiamethoxam by soil enrichment cultures, and the bacterial diversity and plant growth-promoting properties of the cultured isolates[J]. Journal of Environmental Science and Health Part B, Pesticides, Food Contaminants, and Agricultural Wastes, 2014, 49(6): 381-390 (  0) 0) |
| [68] |
Zhang P, Ren C, Sun H W, et al. Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms[J]. Science of the Total Environment, 2018, 615: 59-69 (  0) 0) |
| [69] |
Wang X, Xue L G, Chang S J, et al. Bioremediation and metabolism of clothianidin by mixed bacterial consortia enriched from contaminated soils in Chinese greenhouse[J]. Process Biochemistry, 2019, 78: 114-122 (  0) 0) |
| [70] |
王霞. 噻虫胺降解菌株的分离、鉴定及其在土壤污染修复中的应用基础[D]. 兰州: 兰州交通大学, 2019.
(  0) 0) |
| [71] |
Verma P, Verma P, Sagar R. Variations in N mineralization and herbaceous species diversity due to sites, seasons, and N treatments in a seasonally dry tropical environment of India[J]. Forest Ecology and Management, 2013, 297: 15-26 (  0) 0) |
| [72] |
Feng Y M, Zhang W P, Pang S M, et al. Kinetics and new mechanism of azoxystrobin biodegradation by an Ochrobactrum anthropi strain SH14[J]. Microorganisms, 2020, 8(5): 625 (  0) 0) |
 2022, Vol. 54
2022, Vol. 54