2. 中国科学院大学, 北京 100049
氧化亚氮(N2O)是一种温室气体,其全球变暖潜力是CO2的298倍[1]。由于N2O造成的较强温室效应和臭氧层破坏,使其受到了广泛的关注[2-4]。农业是N2O重要的排放源,由施肥和自然土壤排放的N2O量占全球所有N2O来源的56% ~ 70%[5],因此提高氮肥利用率和减少农业N2O排放变得至关重要。
土壤的硝化和反硝化作用均可以产生N2O[6]。其中,氨单加氧酶编码基因AOA-amoA和AOB-amoA是硝化作用的关键功能基因,亚硝酸盐还原酶编码基因nirK、nirS和N2O还原酶基因nosZ是反硝化作用的关键功能基因[7-8]。硝化抑制剂可以抑制土壤的硝化作用,并且可以有效降低土壤中NO3--N含量,进而减少N2O排放[9-11]。但目前大多数可用的硝化抑制剂均是化学合成,其应用受多种因素的影响[12];同时,合成硝化抑制剂的应用会增加农业生产成本,也可能会造成潜在的环境污染风险[13-14]。
生物硝化抑制剂(Biological Nitrification Inhibitors,BNIs)是一类由植物分泌并具有抑制土壤硝化作用的物质[15-19]。这种天然源的物质比合成硝化抑制剂更环保[20],是现代农业中提高氮肥利用率和减少环境污染的新策略[21-23]。在过去20年中,研究人员从不同植物根系分泌物中鉴定出了不同类型的BNIs。在高粱根系分泌物中鉴定出3种BNIs,分别为3-(4-羟基苯基)丙酸甲酯(methyl 3-(4-hydroxyphenyl) propionate,MHPP)、樱花素(sakuranetin)和高粱酮(sorgoleone)[17-24]。在臂形草(Brachiaria humidicola)根系中分离出臂形草内酯(brachialactone)[25]。在水稻根系分泌物中先后鉴定出2种BNIs,分别是1,9-癸二醇(1,9-D)和丁香酸[18, 26-28]。在玉米根系分泌物中分离出2, 7-dimethoxy-1, 4-naphthoquinone和2-hydroxy-4, 7-dimethoxy-2H-1, 4-benzoxazin-3(4H)-one[29]。研究发现,植物BNIs的分泌量与土壤N2O排放呈显著负相关关系[25],同时,将富含BNIs的牧草覆盖在土壤牛尿斑块上,可以显著降低土壤N2O排放60%[30]。
此外,在一些土壤类型上,MHPP可显著降低土壤N2O排放18.1% ~ 72.2%[31-32];1,9-D可显著平均降低典型土壤N2O排放48%[15]。然而,现有研究均是针对BNIs在调控土壤N2O排放方面的作用,不同BNIs之间对N2O排放的影响差异尚不清楚,且BNIs对N2O减排的微生物学机制也鲜有报道。
红壤作为我国南方典型土壤,由于人为和自然因素,面临着土壤酸化、养分匮乏和流失等多种问题,制约了土壤的绿色健康发展[33]。在红壤上开垦种植水稻以加速红壤熟化,成为发展丘陵地区农业生产的有效途径[34]。虽然在红壤性水稻土上筛选适宜的合成硝化抑制剂来调控土壤N2O排放也有较多报道[35-36],但不同BNIs在该土壤上对N2O排放效果及机制尚不清楚。因此,本研究通过土柱培养试验,比较了3种不同的BNIs(1,9-D、LN(亚麻酸)、MHPP)在红壤性水稻土淹水条件下对N2O排放及硝化、反硝化相关功能基因(AOA-amoA、AOB-amoA、nirK、nirS和nosZ)的影响,并分析了功能基因与N2O排放间的关系,以为BNIs在农田上的精准应用及有效的N2O减排措施制定提供理论依据。
1 材料与方法 1.1 供试材料供试土壤采自中国科学院江西鹰潭农业生态系统国家野外科学观测研究站(28°15′N, 116°55′E),该地区年均气温17.6 ℃,年均降水量为1 795 mm。供试土壤类型为水稻土,质地为砂壤,采自0 ~ 20 cm耕层。采集的土样经风干、过筛(< 2 mm)、混匀,备用,土壤理化性质见表 1。供试1,9-D定制于药明康德新药开发有限公司,纯度大于97%。LN、MHPP和双氰胺(DCD)购买于Sigma公司。
|
|
表 1 试验用土理化性质 Table 1 Physicochemical properties of tested red paddy soil |
试验采用土柱培养方式,土柱高18 cm,内径5 cm,从上至下依次为1 cm空余层、2 cm淹水层、14 cm土肥混合层和1 cm淋溶层。试验开始前1天,先在土柱底部放置多孔挡板和石英砂,然后加入370 g试验用土,压实后浇透水,并放置1 d以使土壤活力达到稳定状态。第2天,将各处理的氮肥和硝化抑制剂溶于蒸馏水后注射入5 cm深的土肥混合层,然后保持2 cm淹水,开始试验。试验每天收集完N2O后再收集一定体积的淋溶液,以模拟田间状态。
试验处理为:①氮肥(尿素,N 200 mg/kg土,记作U);②DCD 20 mg/kg土+氮肥(记作DCD);③1,9-D 100 mg/kg土+氮肥(记作1,9-D);④LN 100 mg/kg土+氮肥(记作LN);④MHPP(100 mg/kg土)+氮肥(记作MHPP)。其中,1,9-D、LN、MHPP和DCD的使用量设置参考其他BNIs的室内培养研究[15, 37-38]。
1.3 土壤N2O排放测定通过使用密闭式静态暗箱–气相色谱法测定N2O。该密闭生长箱内径5 cm,高15 cm,上部封口,下部有凹槽可与土柱扣紧,侧边开孔以便气体的采集,内贴锡纸防止太阳辐射及箱内温度升高。在施肥后连续21 d进行气体采集,分别在盖箱后2 h和4 h时用注射器收集15 mL气体于真空瓶(气袋)中,同时收集3管大气作为空白对照,N2O浓度与用安捷伦7890A气相色谱仪(ECD检测器)测定。
N2O日排放量计算公式 [39]:F(N,µg/kg)=(M/VM)×V×dc/dt×273/(273+T)×P/P0×24。式中:F为N2O的排放通量;M为N2O-N标准状态下的摩尔质量(28 g/mol);VM为气体的摩尔体积,22.4 L/mol;V为密闭静态箱体积(L);dc/dt为采样过程中箱内N2O气体浓度变化率(N,µg/(L×h));T为采样时箱内的平均温度(℃);P为采样箱内气压;P0为标准大气压。试验场地海拔高度为30 m,压强影响较小,实际计算中忽略气压的影响。
1.4 土壤DNA提取和定量PCR土壤细菌DNA使用MoBio PowerSoil DNA试剂盒(美国)进行提取,根据制造商要求进行操作,提取土壤DNA后,使用NanoDrop ND100分光光度计(威尔明顿,美国)评估提取的DNA浓度和质量。使用LightCycler 480实时PCR系统(罗氏诊断公司,曼海姆,德国)进行实时定量PCR测定,使用引物见表 2。10 µL反应混合液中包含5 µL SYBR Premix Ex Taq酶(TaKaRa,东京,日本)、0.4 µL前引物、0.4 µL后引物和0.5µL DNA。将含有目标基因质粒按照10倍浓度梯度进行稀释制成标准曲线。每次循环结束后进行溶解曲线分析以评估定量PCR扩增的特异性。
|
|
表 2 定量PCR的引物及扩增条件 Table 2 Primers sequences and real-time PCR program |
试验数据采用Excel 2021程序和SPSS 20软件进行处理与统计分析,采用单因素方差分析和Duncan法进行显著性检验和多重比较,采用Pearson法对各变量进行相关性分析。采用Origin 2022软件作图。
2 结果与分析 2.1 不同BNIs与DCD对土壤N2O排放的影响由图 1A可以发现,所有施肥处理的土壤N2O日排放量在施肥后3 d时达到峰值,在施肥7 d后趋于稳定。但与U处理相比,不同BNIs处理可平均降低土壤N2O日排放峰值40.1%,远高于DCD处理的19.6%。同时,在不同BNIs处理之间,1,9-D处理对N2O日排放峰值的抑制作用最好(44.5%),其次是MHPP处理(43.9%)和LN处理(31.8%)。从土壤N2O排放累积量来看(图 1B),LN和DCD处理对N2O排放量没有显著抑制作用,1,9-D和MHPP处理可以显著抑制土壤N2O排放量,其中,1,9-D处理可以减排22.5%,MHPP处理可以减排17.4%。由此可见,在淹水条件下,1,9-D对红壤性水稻土N2O的减排作用优于MHPP、LN和DCD。

|
(图中不同小写字母表示处理间差异在P<0.05水平显著,下同) 图 1 不同BNIs与DCD与对N2O排放的影响 Fig. 1 Effects of different BNIs and DCD on N2O emissions |
通过荧光定量PCR测定土壤硝化和反硝化过程关键功能基因丰度发现,与U处理相比,不同BNIs处理可平均显著降低土壤氨氧化古菌(AOA)基因丰度24.1%,远高于DCD处理的7.9%(图 2A,图 2B);其中,1,9-D处理对AOA基因丰度的抑制作用(37.3%)远高于LN处理(25.3%)和MHPP处理(9.5%)。对于氨氧化细菌(AOB)基因丰度,DCD处理的抑制率为33.3%,高于BNIs处理;而不同BNIs处理间,1,9-D处理的抑制作用(23.7%)高于LN处理(1.2%)和MHPP处理(11.5%)。
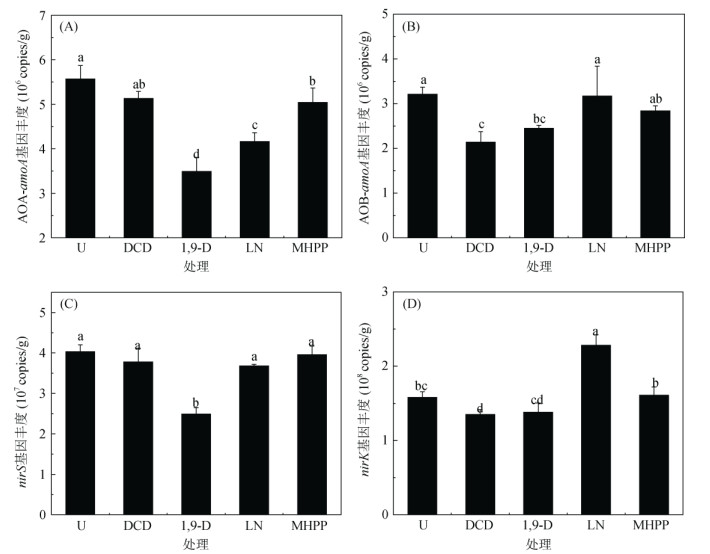
|
图 2 不同BNIs和DCD对土壤硝化(AOA-amoA、AOB-amoA)和反硝化功能基因(nirS、nirK)丰度的影响 Fig. 2 Effects of different BNIs and DCD on gene abundances of soil nitrification (AOA-amoA, AOB-amoA) and denitrification functional genes (nirS, nirK) |
对于反硝化基因,不同BNIs和DCD处理则表现出不同的趋势(图 2C,图 2D)。与U处理相比,1,9-D处理显著降低土壤nirS基因丰度38.2%,LN、MHPP和DCD处理则没有显著的抑制作用。DCD处理显著降低土壤nirK基因丰度33.3%,1,9-D和MHPP处理没有显著的影响,而LN处理显著提高nirK基因丰度54.1%。
2.3 不同BNIs和DCD对nosZ基因丰度及其在硝化、反硝化相关功能基因中占比的影响由图 3A可知,BNIs处理对土壤nosZ基因丰度的促进作用远高于DCD处理。在不同BNIs处理中,1,9-D和MHPP处理可以显著提高nosZ基因丰度18.5% 和34.4%,LN处理则显著降低nosZ基因丰度52.9%。通过nosZ基因丰度与硝化或反硝化细菌功能基因丰度的比值(图 3B ~ 3D),可以看出BNIs处理的nosZ/(AOA-amoA+ AOB-amoA)、nosZ/(nirS+nirK)和nosZ/(AOA-amoA+ AOB-amoA+nirS+nirK)平均比值均高于DCD处理。与U处理相比,1,9-D和MHPP处理均显著提高3组比值,分别为81.2% 和47.8%、31.3% 和19.3%、32.9% 和21.5%,1,9-D处理的3组比值均高于MHPP处理;LN和DCD处理则显著降低3组比值,分别降低14.7% 和47.8%、24.1% 和66.3%、24.1% 和65.8%,其中,DCD处理的3组比值均高于LN处理,但均低于1,9-D和MHPP处理。

|
图 3 不同BNIs与DCD对nosZ基因丰度,nosZ/(AOA-amoA+ AOB-amoA)、nosZ/(nirS+nirK)和nosZ/(AOA-amoA+ AOB-amoA+nirS+nirK)比值的影响 Fig. 3 Effects of different BNIs and DCD on nosZ gene abundances, nosZ/(AOA-amoA+ AOB-amoA), nosZ/(nirS+nirK), and nosZ/(AOA-amoA+ AOB-amoA+nirS+nirK) ratios |
对土壤N2O排放与不同功能基因进行相关性分析,发现在红壤性水稻土淹水条件下,土壤N2O排放总量与硝化过程功能基因AOA-amoA丰度呈显著正相关(R2=0.40,P<0.05),与AOB-amoA丰度则无显著相关性(图 4)。对于反硝化功能基因,土壤N2O排放总量与nirS丰度呈显著正相关(R2=0.38,P<0.05),与nosZ丰度呈显著负相关(R2=0.43,P<0.05),与nirK丰度无显著相关关系。值得关注的是,土壤N2O排放总量与nosZ/(AOA-amoA+AOB-amoA)相关性最高(R2=0.71,P<0.01),与nosZ/(AOA-amoA+AOB-amoA+nirS+nirK)(R2=0.47,P<0.01)和nosZ/(nirS+nirK)(R2=0.46,P<0.01)呈显著负相关关系。
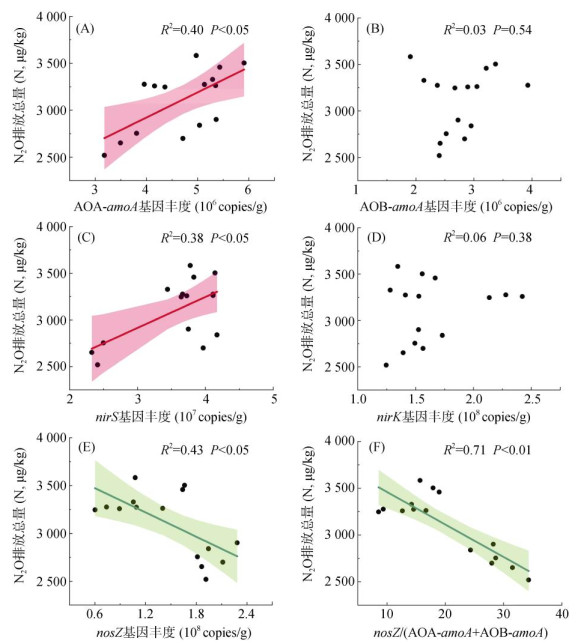
|
图 4 土壤N2O排放总量与硝化、反硝化功能基因丰度和相关功能基因比值的相关性 Fig. 4 Correlation analysis of total soil N2O emissions with abundances of nitrification and denitrification functional genes and the ratios of related functional genes |
生物硝化抑制剂(BNIs)作为一种可以抑制土壤硝化作用的物质,被认为具有调控土壤N2O排放的潜力[15, 21-23, 31, 44]。本研究中,DCD虽然可以降低红壤性水稻土淹水条件下土壤N2O日排放峰值,但对N2O排放总量没有显著的影响,这与以往DCD在红壤上的研究结果一致[15]。但DCD在碱性潮土和水稻土上
却表现出较好的N2O减排趋势[45-48],这说明DCD的减排作用可能与土壤类型有关。本研究中,不同BNIs均比DCD具有更低的N2O日排放峰值和排放总量,在不同BNIs中以1,9-D对N2O减排作用最好,其次是MHPP和LN。其中,1,9-D可以降低N2O排放总量44.5%,略低于Lu等[15]研究;MHPP可以显著抑制土壤N2O排放总量43.9%,但减排效果弱于Lan等[31]研究,这可能是因为本研究中MHPP施用量仅为100 mg/kg土,低于Lan等[31]研究的施用量500 mg/kg土,这预示着MHPP调控N2O的排放可能具有浓度效应,值得今后深入研究。LN虽然可以显著降低土壤N2O日排放的峰值,但对土壤N2O排放的总量却没有显著的抑制作用,这与LN在高硝化土壤中的N2O减排作用结果一致[38]。
虽然不同BNIs均可以降低N2O排放,但BNIs之间的减排作用却有较大的差异。本研究中,仅有1,9-D和MHPP对土壤N2O具有显著的减排作用,而且1,9-D对N2O的减排作用优于MHPP。一方面是因为1,9-D处理比MHPP处理具有更低的AOA-amoA、AOB-amoA和nirS基因丰度;另一方面,1,9-D处理比MHPP处理有更高的nosZ/(AOA-amoA+AOB-amoA)、nosZ/(nirS+nirK)和nosZ/(AOA-amoA+AOB-amoA+nirS+nirK)比值。而nosZ基因丰度与硝化、反硝化功能基因丰度的比值可决定部分N2O净产生水平[49],这说明1,9-D比MHPP能更好地同时调控硝化和反硝化过程,进而产生更少的土壤N2O排放。同时,由于本实验体系为了模拟田间真实情况会收集淋溶液,而MHPP作为水溶性物质[17],易随水流失,从而可能减弱了其对土壤硝化过程的抑制作用及减排N2O的效果;1,9-D作为水稻根系分泌的脂溶性物质[18],不易在土壤中移动,为更好更长久地调控N2O排放提供了可能性。
硝化过程中的AOA和AOB硝化菌基因丰度与土壤N2O排放密切相关[6]。本研究中,淹水条件下红壤性水稻土的N2O排放总量与AOA硝化菌基因丰度呈显著正相关关系(R2=0.40,P<0.05),与AOB硝化菌基因丰度无显著相关性,这与Ji等[49]和Yang等[50]的研究结果相似。这可能是因为AOA数量在大多数土壤中优于AOB,使得土壤硝化作用产生的N2O总排放量中很大一部分来源于AOA过程[51-52];同时,在酸性土壤中,土壤微生物优势种群会发生转变,进而使N2O排放与土壤AOA基因丰度具有更好的交互性[15]。nirS、nirK和nosZ均是反硝化过程中产生N2O的主要功能基因[53-54]。本研究发现,土壤N2O排放总量与nirS和nosZ基因丰度呈显著正相关关系,与nirK基因丰度无显著相关性。这说明在红壤淹水体系下,除了硝化过程,反硝化过程在调控土壤N2O排放中也扮演重要的角色[55]。此外,土壤N2O排放总量还与nosZ基因丰度相关比值呈显著正相关关系,进一步证实了淹水条件下1,9-D和MHPP对土壤N2O的调控是硝化和反硝化过程共同作用的结果。然而以往的研究大都关注了AOA和AOB这些硝化功能基因在N2O排放中的作用[15, 31]。本研究中,尽管1,9-D和MHPP均有抑制AOA-amoA和AOB-amoA基因丰度的趋势,但这两种BNIs对N2O的减排主要是通过抑制前者实现,因为AOA-amoA的丰度与N2O减排呈正相关。研究表明,BNIs对AOA的亲和性普遍比AOB强[15, 37]。1,9-D和MHPP处理的nosZ基因丰度及相关比值的促进表明这两种BNIs能够增强N2O到N2的还原过程。Florio等[56]研究发现高BNIs活性的森林树种比无BNIs活性的树种更能显著抑制N2O排放,这与其能促进nosZ基因丰度有关。然而BNIs影响nosZ还原微生物的机理还尚不清楚,有待进一步研究。
4 结论在红壤性水稻土淹水条件下,生物硝化抑制剂1,9-D和MHPP能显著抑制N2O排放峰值和总量,而LN和DCD无显著影响。这些生物硝化抑制剂和化学合成硝化抑制剂对N2O排放的效果差异与微生物硝化与反硝化过程密切相关。AOB-amoA、nirS和nosZ基因丰度与土壤N2O排放主要关联。1,9-D抑制了AOA、AOB、nirS型反硝化微生物,促进了N2O还原菌的生长,而MHPP主要改变了AOA微生物和N2O还原菌的生长。可见,施用生物硝化抑制剂1,9-D和MHPP将有望成为红壤性水稻土中提高氮素利用率与减少N2O排放的潜力措施。然而,未来这些BNIs的减排效果需要在田间条件下通过更长的试验周期进行验证。
| [1] |
Solomon S C, Qin D, Manning M R, et al. Climate change: The physical science basis. Contribution of working group i to the fourth assessment report of the intergovernmental panel on climate change[J]. Summary for Policymakers, 2007 (  0) 0) |
| [2] |
Smith K A, Mosier A R, Crutzen P J, et al. The role of N2O derived from crop-based biofuels, and from agriculture in general, in Earth's climate[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2012, 367(1593): 1169-1174 DOI:10.1098/rstb.2011.0313 (  0) 0) |
| [3] |
Sun H J, Zhang H L, Powlson D, et al. Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor 2-chloro-6-(trichloromethyl)-pyridine[J]. Field Crops Research, 2015, 173: 1-7 DOI:10.1016/j.fcr.2014.12.012 (  0) 0) |
| [4] |
Xu J Y, Min J, Sun H J, et al. Biostimulants decreased nitrogen leaching and NH3 volatilization but increased N2O emission from plastic-shed greenhouse vegetable soil[J]. Environmental Science and Pollution Research, 2022, 29(4): 6093-6102 DOI:10.1007/s11356-021-16039-y (  0) 0) |
| [5] |
Syakila A, Kroeze C. The global nitrous oxide budget revisited[J]. Greenhouse Gas Measurement and Management, 2011, 1(1): 17-26 DOI:10.3763/ghgmm.2010.0007 (  0) 0) |
| [6] |
Liu Y R, Delgado-Baquerizo M, Trivedi P, et al. Species identity of biocrust-forming lichens drives the response of soil nitrogen cycle to altered precipitation frequency and nitrogen amendment[J]. Soil Biology and Biochemistry, 2016, 96: 128-136 DOI:10.1016/j.soilbio.2016.01.021 (  0) 0) |
| [7] |
Ligi T, Truu M, Truu J, et al. Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex[J]. Ecological Engineering, 2014, 72: 47-55 DOI:10.1016/j.ecoleng.2013.07.015 (  0) 0) |
| [8] |
Xu H J, Wang X H, Li H, et al. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape[J]. Environmental Science & Technology, 2014, 48(16): 9391-9399 (  0) 0) |
| [9] |
Min J, Sun H J, Kronzucker H J, et al. Comprehensive assessment of the effects of nitrification inhibitor application on reactive nitrogen loss in intensive vegetable production systems[J]. Agriculture, Ecosystems & Environment, 2021, 307: 107227 (  0) 0) |
| [10] |
沈晓忆, 夏围围, 张洁, 等. 硝化抑制剂与尿素配施对旱地土壤温室气体排放及硝化微生物的影响[J]. 土壤, 2021, 53(3): 512-521 DOI:10.13758/j.cnki.tr.2021.03.010 (  0) 0) |
| [11] |
孙海军, 闵炬, 施卫明, 等. 硝化抑制剂影响小麦产量、N2O与NH3排放的研究[J]. 土壤, 2017, 49(5): 876-881 DOI:10.13758/j.cnki.tr.2017.05.003 (  0) 0) |
| [12] |
Abalos D, Jeffery S, Sanz-Cobena A, et al. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency[J]. Agriculture, Ecosystems & Environment, 2014, 189: 136-144 (  0) 0) |
| [13] |
Sun H J, Min J, Shi W M, et al. Effects of nitrification inhibitor application on wheat grain yield, N2O emission and NH3 volatilization[J]. Environmetal Science and Pollution Research, 2017, 49(5): 876-881 (  0) 0) |
| [14] |
Woodward E E, Hladik M L, Kolpin D W. Nitrapyrin in streams: The first study documenting off-field transport of a nitrogen stabilizer compound[J]. Environmental Science & Technology Letters, 2016, 3(11): 387-392 (  0) 0) |
| [15] |
Lu Y F, Zhang X N, Jiang J F, et al. Effects of the biological nitrification inhibitor 1,9-decanediol on nitrification and ammonia oxidizers in three agricultural soils[J]. Soil Biology and Biochemistry, 2019, 129: 48-59 DOI:10.1016/j.soilbio.2018.11.008 (  0) 0) |
| [16] |
Subbarao G V, Ishikawa T, Ito O, et al. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola[J]. Plant and Soil, 2006, 288(1): 101-112 (  0) 0) |
| [17] |
Subbarao G V, Nakahara K, Ishikawa T, et al. Biological nitrification inhibition (BNI) activity in sorghum and its characterization[J]. Plant and Soil, 2013, 366(1): 243-259 (  0) 0) |
| [18] |
Sun L, Lu Y F, Yu F W, et al. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency[J]. The New Phytologist, 2016, 212(3): 646-656 DOI:10.1111/nph.14057 (  0) 0) |
| [19] |
Zhang X N, Lu Y F, Yang T, et al. Factors influencing the release of the biological nitrification inhibitor 1,9-decanediol from rice (Oryza sativa L.) roots[J]. Plant and Soil, 2019, 436(1): 253-265 (  0) 0) |
| [20] |
陆玉芳, 施卫明. 根际化学信号物质与土壤养分转化[J]. 生物技术通报, 2020, 36(9): 14-24 (  0) 0) |
| [21] |
Coskun D, Britto D T, Shi W M, et al. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition[J]. Nature Plants, 2017, 3: 17074 DOI:10.1038/nplants.2017.74 (  0) 0) |
| [22] |
曾后清, 朱毅勇, 王火焰, 等. 生物硝化抑制剂——一种控制农田氮素流失的新策略[J]. 土壤学报, 2012, 49(2): 382-388 (  0) 0) |
| [23] |
陆玉芳, 施卫明. 生物硝化抑制剂的研究进展及其农业应用前景[J]. 土壤学报, 2021, 58(3): 545-557 (  0) 0) |
| [24] |
Zakir H A K M, Subbarao G V, Pearse S J, et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by Sorghum (Sorghum bicolor)[J]. The New Phytologist, 2008, 180(2): 442-451 DOI:10.1111/j.1469-8137.2008.02576.x (  0) 0) |
| [25] |
Subbarao G V, Nakahara K, Hurtado M P, et al. Evidence for biological nitrification inhibition in Brachiaria pastures[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(41): 17302-17307 (  0) 0) |
| [26] |
Lu Y F, Zhang X N, Ma M K, et al. Syringic acid from rice as a biological nitrification and urease inhibitor and its synergism with 1,9-decanediol[J]. Biology and Fertility of Soils, 2022, 58(3): 277-289 DOI:10.1007/s00374-021-01584-y (  0) 0) |
| [27] |
杨婷, 陆玉芳, 马明坤, 等. 超声提取-气相色谱法测定土壤1,9-癸二醇[J]. 土壤学报, 2021, 58(4): 968-977 (  0) 0) |
| [28] |
张晓楠, 陆玉芳, 杨婷, 等. 水稻生物硝化抑制剂1,9-癸二醇的定量方法优化[J]. 土壤, 2020, 52(6): 1152-1157 DOI:10.13758/j.cnki.tr.2020.06.008 (  0) 0) |
| [29] |
Otaka J, Subbarao G V, Ono H, et al. Biological nitrification inhibition in maize—Isolation and identification of hydrophobic inhibitors from root exudates[J]. Biology and Fertility of Soils, 2022, 58(3): 251-264 DOI:10.1007/s00374-021-01577-x (  0) 0) |
| [30] |
Byrnes R C, Nùñez J, Arenas L, et al. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches[J]. Soil Biology and Biochemistry, 2017, 107: 156-163 DOI:10.1016/j.soilbio.2016.12.029 (  0) 0) |
| [31] |
Lan T, Huang Y X, Song X, et al. Biological nitrification inhibitor co-application with urease inhibitor or biochar yield different synergistic interaction effects on NH3 volatilization, N leaching, and N use efficiency in a calcareous soil under rice cropping[J]. Environmental Pollution, 2022, 293: 118499 DOI:10.1016/j.envpol.2021.118499 (  0) 0) |
| [32] |
Zhang M, Fan C H, Li Q L, et al. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system[J]. Agriculture, Ecosystems & Environment, 2015, 201: 43-50 (  0) 0) |
| [33] |
谢正苗, 吕军, 俞劲炎, 等. 红壤退化过程与生态位的研究[J]. 应用生态学报, 1998, 9(6): 669-672 (  0) 0) |
| [34] |
邓铁金, 樊友安, 周任发. 红壤性水稻土的形成过程特点及其肥力演变[J]. 土壤学报, 1985, 22(1): 1-12 (  0) 0) |
| [35] |
崔磊, 李东坡, 武志杰, 等. 不同硝化抑制剂对红壤氮素硝化作用及玉米产量和氮素利用率的影响[J]. 应用生态学报, 2021, 32(11): 3953-3960 (  0) 0) |
| [36] |
张昊青, 赵学强, 张玲玉, 等. 石灰和双氰胺对红壤酸化和硝化作用的影响及其机制[J]. 土壤学报, 2021, 58(1): 169-179 (  0) 0) |
| [37] |
Lan T, Xie N, Chen C, et al. Effects of biological nitrification inhibitor in regulating NH3 volatilization and fertilizer nitrogen recovery efficiency in soils under rice cropping[J]. Science of the Total Environment, 2022, 838: 155857 DOI:10.1016/j.scitotenv.2022.155857 (  0) 0) |
| [38] |
Ma Y, Jones D L, Wang J Y, et al. Relative efficacy and stability of biological and synthetic nitrification inhibitors in a highly nitrifying soil: Evidence of apparent nitrification inhibition by linoleic acid and linolenic acid[J]. European Journal of Soil Science, 2021, 72(6): 2356-2371 DOI:10.1111/ejss.13096 (  0) 0) |
| [39] |
Gao X S, Deng O P, Ling J, et al. Effects of controlled-release fertilizer on nitrous oxide and nitric oxide emissions during wheat-growing season: Field and pot experiments[J]. Paddy and Water Environment, 2018, 16(1): 99-108 DOI:10.1007/s10333-017-0619-6 (  0) 0) |
| [40] |
Francis C A, Roberts K J, Beman J M, et al. Ubiquity and diversity of ammonia-oxidizing Archaea in water columns and sediments of the ocean[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(41): 14683-14688 (  0) 0) |
| [41] |
Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations[J]. Applied and Environmental Microbiology, 1997, 63(12): 4704-4712 DOI:10.1128/aem.63.12.4704-4712.1997 (  0) 0) |
| [42] |
Hallin S, Lindgren P E. PCR detection of genes encoding nitrite reductase in denitrifying bacteria[J]. Applied and Environmental Microbiology, 1999, 65(4): 1652-1657 DOI:10.1128/AEM.65.4.1652-1657.1999 (  0) 0) |
| [43] |
Henry S, Bru D, Stres B, et al. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils[J]. Applied and Environmental Microbiology, 2006, 72(8): 5181-5189 DOI:10.1128/AEM.00231-06 (  0) 0) |
| [44] |
Subbarao G V, Ito O, Sahrawat K L, et al. Scope and strategies for regulation of nitrification in agricultural systems—Challenges and opportunities[J]. Critical Reviews in Plant Sciences, 2006, 25(4): 303-335 DOI:10.1080/07352680600794232 (  0) 0) |
| [45] |
Ding W X, Yu H Y, Cai Z C. Impact of urease and nitrification inhibitors on nitrous oxide emissions from fluvo-aquic soil in the North China Plain[J]. Biology and Fertility of Soils, 2011, 47(1): 91-99 DOI:10.1007/s00374-010-0504-6 (  0) 0) |
| [46] |
Lan T, Han Y, Roelcke M, et al. Effects of the nitrification inhibitor dicyandiamide (DCD) on gross N transformation rates and mitigating N2O emission in paddy soils[J]. Soil Biology and Biochemistry, 2013, 67: 174-182 DOI:10.1016/j.soilbio.2013.08.021 (  0) 0) |
| [47] |
Li X L, Zhang G B, Xu H, et al. Effect of timing of joint application of hydroquinone and dicyandiamide on nitrous oxide emission from irrigated lowland rice paddy field[J]. Chemosphere, 2009, 75(10): 1417-1422 DOI:10.1016/j.chemosphere.2009.02.006 (  0) 0) |
| [48] |
王艳群, 李迎春, 彭正萍, 等. 氮素配施双氰胺对冬小麦-夏玉米轮作系统N2O排放的影响及效益分析[J]. 应用生态学报, 2015, 26(7): 1999-2006 (  0) 0) |
| [49] |
Ji C, Li S Q, Geng Y J, et al. Decreased N2O and NO emissions associated with stimulated denitrification following biochar amendment in subtropical tea plantations[J]. Geoderma, 2020, 365: 114223 DOI:10.1016/j.geoderma.2020.114223 (  0) 0) |
| [50] |
Yang L Q, Zhu G D, Ju X T, et al. How nitrification-related N2O is associated with soil ammonia oxidizers in two contrasting soils in China?[J]. Science of the Total Environment, 2021, 770: 143212 DOI:10.1016/j.scitotenv.2020.143212 (  0) 0) |
| [51] |
Jung M Y, Well R, Min D, et al. Isotopic signatures of N2O produced by ammonia-oxidizing Archaea from soils[J]. The ISME Journal, 2014, 8(5): 1115-1125 DOI:10.1038/ismej.2013.205 (  0) 0) |
| [52] |
Schleper C. Ammonia oxidation: Different niches for bacteria and Archaea?[J]. The ISME Journal, 2010, 4(9): 1092-1094 DOI:10.1038/ismej.2010.111 (  0) 0) |
| [53] |
Chèneby D, Hartmann A, Hénault C, et al. Diversity of denitrifying microflora and ability to reduce N2O in two soils[J]. Biology and Fertility of Soils, 1998, 28(1): 19-26 DOI:10.1007/s003740050458 (  0) 0) |
| [54] |
Cytryn E, Levkovitch I, Negreanu Y, et al. Impact of short-term acidification on nitrification and nitrifying bacterial community dynamics in soilless cultivation media[J]. Applied and Environmental Microbiology, 2012, 78(18): 6576-6582 DOI:10.1128/AEM.01545-12 (  0) 0) |
| [55] |
Estavillo J, Merino P, Pinto M, et al. Short term effect of ploughing a permanent pasture on N2O production from nitrification and denitrification[J]. Plant and Soil, 2002, 239(2): 253-265 DOI:10.1023/A:1015062304915 (  0) 0) |
| [56] |
Florio A, Marechal M, Legout A, et al. Influence of biological nitrification inhibition by forest tree species on soil denitrifiers and N2O emissions[J]. Soil Biology and Biochemistry, 2021, 155: 108164 DOI:10.1016/j.soilbio.2021.108164 (  0) 0) |
2. University of Chinese Academy of Sciences, Beijing 100049, China
 2024, Vol. 56
2024, Vol. 56


